What we investigate
We investigate acoustic cavitation processes by advancing experimental techniques to capture ultrafast fluid flows at microscale. From contrast-enhanced medical ultrasound to ablation by focusing shock waves, we aim at identifying the physical mechanisms behind cavitation-induced effects, predicting them through theoretical modelling and controlling them in use primarily in therapeutic, fluid processing and fluidic transport applications.
KEYWORDS
cavitation, fluid dynamics, medical ultrasound, ultrasound contrast agents
Our research in more detail
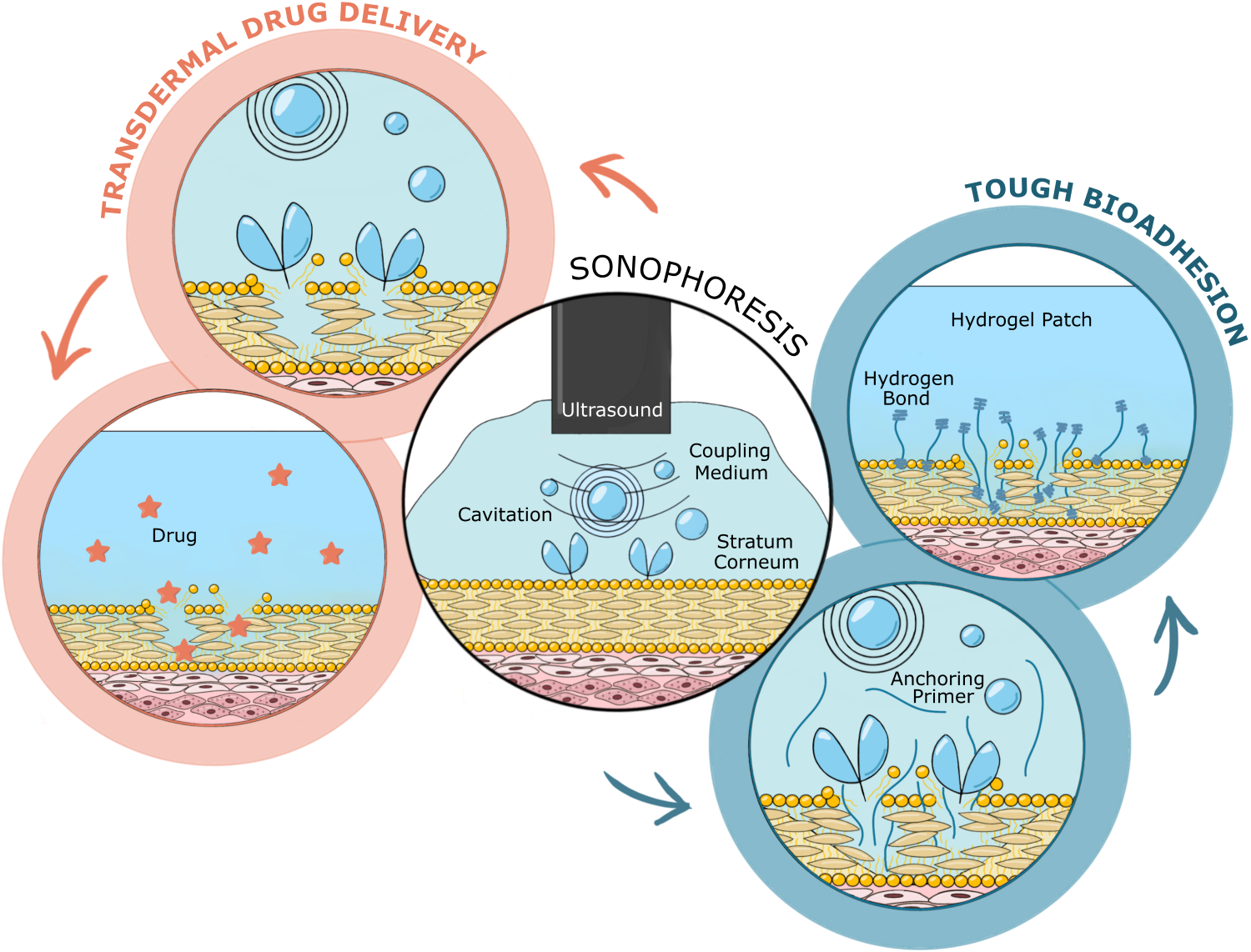
Sonophoresis is an ultrasound-based strategy used to enhance tough bioadhesion in bioadhesive technologies and to increase skin permeability for transdermal drug delivery, allowing drug molecules, including larger macromolecules, to effectively cross the stratum corneum. The method holds great potential in several areas, including therapeutic applications (targeted drug delivery, dermatological treatments, gene delivery, vaccine delivery, pain management and wound healing) and transdermal diagnostic applications (blood analyte quantification). Although preclinical studies have demonstrated successful applications of sonophoresis protocols, the exact biophysical mechanism underlying sonophoresis remains poorly understood, limiting its translation into the clinic. The scope of our ongoing research is to design appropriate ultrasound driving and coupling media that enhance sound-driven mechanisms (in addition to cavitation, also thermal effects and convective transport) and thereby to optimise the efficacy of sonophoresis. We approach the problem by characterising the acoustic cavitation activity in different coupling media and the resulting microdamage enhancing skin permeability. Currently we work at a highly fundamental level, but are interested in bridging our findings in the underlying physics to applications in drug delivery and tough bioadhesion.
Selected publications
SKINTEGRITY.CH Principal Investigators are in bold:
- Ma Z, Bourquard C, Gao Q, Jiang S, De Iure-Grimmel T, Huo R, Li X, He Z, Yang Z, Yang G, Wang Y, Lam E, Gao Z-H, Supponen O* and Li J* (2022). Controlled tough bioadhesion mediated by ultrasound. Science, 377(6607), 751-755. *corresponding authors
- Shakya G, Cattaneo M, Guerriero G, Prasanna A, Fiorini S and Supponen O (2024). Ultrasound-responsive microbubbles and nanodroplets: A pathway to targeted drug delivery. Adv Drug Deliv Rev, 206, 115178.
- Cattaneo M, Guerriero G, Shakya G, Krattiger LA, Paganella LG, Narciso ML and Supponen O (2025). Cyclic jetting enables microbubble-mediated drug delivery. Nat Phys, in press (arXiv:2410.08990).
